What Causes of Aging The Hallmarks of Aging
What causes of aging-the Hallmarks of Aging
In the past two decades, we have learned more about aging than the previous 2,000 years combined. Aging is caused by many processes besides from DNA damage. These processes are called the hallmarks of aging. Insight learning on hallmarks of aging is crucial and how to identify and reverse them in the upcoming articles.Scientists have begun to identify and categorize the cellular and molecular hallmarks of aging [López-Otín et al. 2013 & 2023]. The hallmarks of Aging or the hallmarks of Senescence, are the biological and chemical changes that occur to your body cells, tissues, and organs when you age. These changes negatively affect your body’s systems and lead to progressive loss of function. Moreover, this decline increases the risk that you will develop diabetes, cancer, heart diseases, or cognitive disorders.
The 12 hallmarks of aging provide a framework for understanding the complex biological processes that contribute to our bodies breaking down over time. While these hallmarks are all interconnected, they each have unique impacts on our health and well-being.
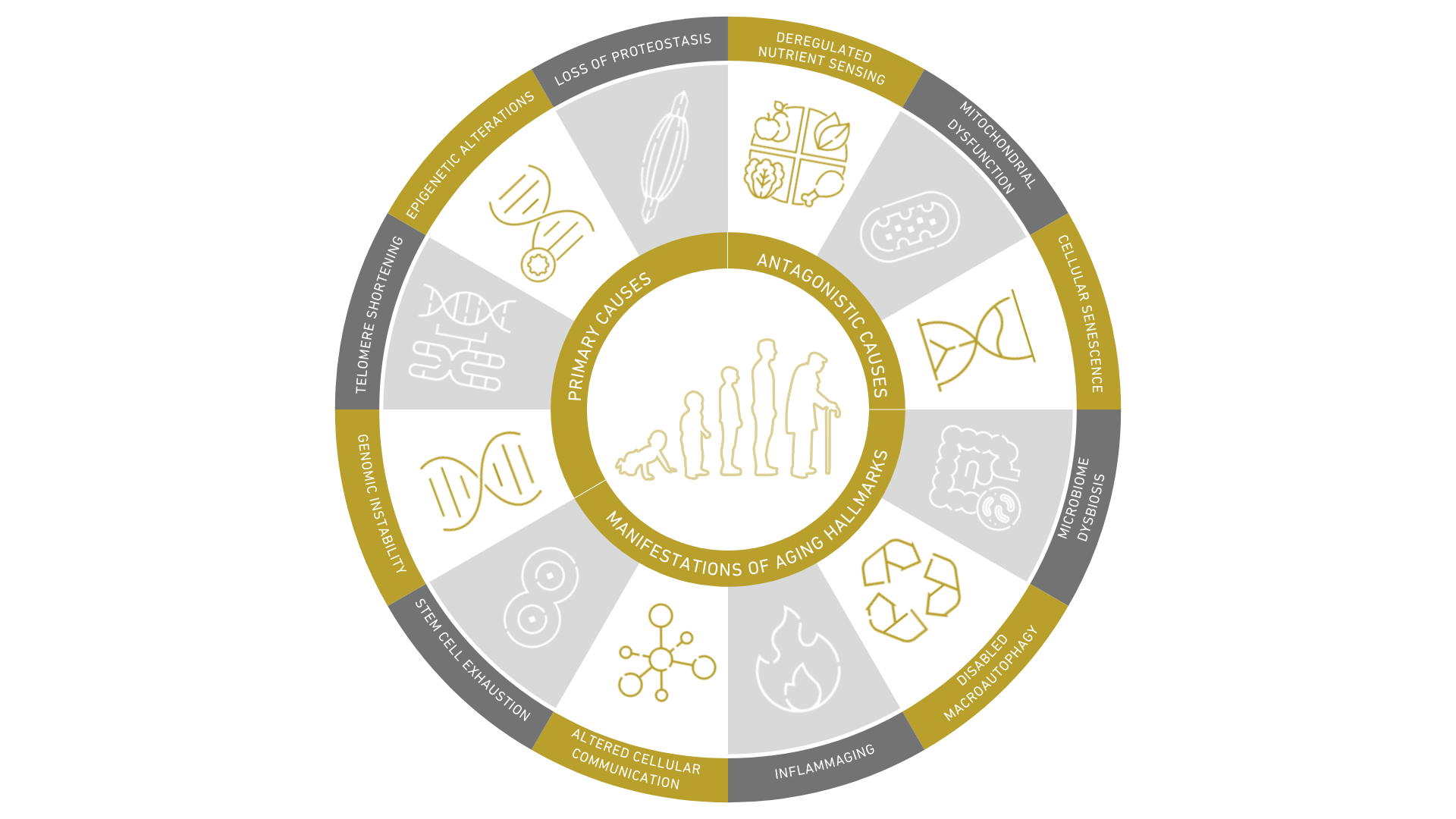
The Hallmarks of Aging:
1. Genomic instability
Our genetic blueprint-DNA fails to remain structurally intact or replicated over time. This genomic instability can lead to DNA damage, which can in turn lead to a variety of health problems.
Our genomic DNA, is constantly being damaged by external and internal factors. These damaging factors include UV radiation from sunlight or reactive oxygen species produced in our mitochondria. It is estimated that our DNA is damaged up to a million times a day. Most of this damage is repaired immediately because cells have efficient detection and repair mechanisms. However, these repair processes are not perfect and a small percentage of damage remains unrepaired. These DNA damage accumulates as we age, which can have several adverse effects. DNA mutations increase the risk of tumor growth, so our risk of cancer increases with age [Roos et al. 2016].
2. Telomere degradation/shortening
Telomeres are the protective caps at the ends of the chromosomes in the human genome. They are like the closed end of a shoelace and keep our chromosomes intact. With each cell division, the telomeres become progressively shorter, and when a certain length is reached, cells enter a resting phase and stop dividing. These cells can then die or even cause inflammation, which accelerates the aging process and triggers disease [Aubert et al. 2008].
Telomere shortening is part of the natural process of the cell cycle. However, some factors accelerate the attrition rate – oxidative stress, inflammation, and chronic stress. They lead to a shorter cell cycle, and thus negatively affect your biological age and longevity.
Telomeres are maintained by an enzyme called telomerase, deficiency of which has been associated with the premature development of several diseases and the loss of tissue regenerative capacity.
3. Epigenetic alterations/changes
Our genome consists of more than 3 billion letters, called nucleotide base pairs, which encode the blueprint of our body. However, the information in the DNA is not only stored in the base pairs, but also in chemical modifications to these letters and to the histone proteins that package our DNA. The sum of these chemical modifications is known as the epigenome. Unlike the genetically encoded information, which is very stable, the epigenome is very dynamic and changes in response to diet, drugs or stress, allowing the cell to adapt to environmental changes. The epigenome also changes with age [Zhang et al. 2020]. A particular modification called DNA methylation is important in this context. Our DNA carries millions of small methyl groups, and this pattern changes with age in a tissue-specific way.
Surprisingly, the DNA methylation pattern of only 350 methylation sites is sufficient to predict a person’s biological age [Horvath 2013]. This so-called ‘epigenetic clock’ has become an important tool as a biomarker to assess whether a particular intervention will have a positive effect on human health span.
This epigenetic alterations are activated or deactivated by factors like enzymes, lifestyle, environments, and behavior. Epigenetic alterations lead to a change in the products your genes produce, without affecting the DNA sequence. However, its plays a crucial role in deteriorated cellular functions you can see in aging and age-related diseases.
4. Loss of proteostasis
Proteins are the most important molecules in our cells, catalysing most biochemical reactions and being important for cell signaling and stability. For properly cell function, proteins must be kept in good condition, a process known as protein homeostasis. To maintain proteostasis, cells have several systems that regulate protein synthesis, folding and degradation. Misfolded and damaged proteins are mainly degraded by the proteasome or by a recycling process called autophagy.
The aging process is characterized by a loss of proteostasis leading to an accumulation of damaged and non-functional proteins. Misfolded proteins can clump together to form aggregates, a characteristic feature of many age-related neurodegenerative diseases such as Alzheimer's and Parkinson's [Hartl et al. 2017].
5. Deregulated nutrients sensing
Our cells need to link their growth and metabolism to the availability of nutrients. Therefore they have nutrient-sensing pathways that sense the nutrient status of the environment, either through hormones or specific nutrient components and adjust cell metabolism accordingly. The insulin and mTOR pathways together form a central nutrient-sensing network within the cell, which has also been linked to the beneficial effects of Dietary Restriction [Swovick et al. 2018]. Interestingly, genetic or pharmacological inhibition of the pathways extends lifespan in a variety of animals, making them a good target for anti-aging drug development [Castillo-Quan et al. 2019].
Our body’s ability to detect and respond to nutrients like glucose, amino acids or fatty acids becomes disrupted. This situation can lead to metabolic dysfunction which is the main cause of conditions such as diabetes and obesity and negatively affects epigenetics.
6. Mitochondrial dysfunction
Mitochondria are small organelles in the cell that are not only the ‘cellular power plants’ but also form a central hub for metabolic processes in the cell. They use oxygen to produce energy in a process called mitochondrial respiration. An important feature of mitochondria is that they contain their own DNA, called mtDNA, which codes for the proteins needed for respiration. An important finding involving mitochondria in the aging process was that mice with a high mutation rate in their mtDNA, known as mtDNA mutator mice, have a short lifespan and show signs of premature ageing [Vermulst et al. 2008].
Also mitochondria are the main source of reactive oxygen species (ROS), which are produced as a by-product of mitochondrial respiration. These free radicals can damage other macromolecules such as DNA, lipids and proteins and are therefore potentially harmful to the cell and accelerating aging process.
7. Cellular senescence
Cellular senescence is a process where cells stop dividing and take on a zombie-like state, in which they are neither living nor dead. This process is involved in aging and is thought to be a contributing factor to age-related diseases. Senescent cells produce harmful chemicals that can damage surrounding cells, leading to inflammation, tissue damage and additional senescent cells. The accumulation of senescent cells is thought to be a major contributor to the aging process.
When we get older, more and more senescent cells start to appear everywhere in our tissues. Senescent cells are called “zombie cells”: these cells should normally have died, but they keep sticking around. Senescent cells are previously healthy cells that have accumulated a lot of damage. Normally, they should kill themselves because of this damage, but they don’t. They linger, secreting harmful molecules, including inflammatory cytokines, growth factors and other molecules. Importantly, senescent cells also negatively affect surrounding cells, contributing to impaired organ function.
8. Exhaustion of stem cells
Stem cells have the ability to divide and differentiate into different cell types. They play a vital role in keeping our organs and body healthy. Aging negatively affects stem cells in many ways, and stem cell ageing itself is thought to contribute to tissue aging, especially in tissues where cells renew frequently. Stem cells can be lost during aging, leading to stem cell depletion and a reduced ability to repair organ damage [Goodell & Rando 2015].
Interestingly, the aging of stem cells was long thought to be irreversible, but recent research suggests that it may be possible to rejuvenate old stem cells. For example, it has been shown that injecting blood plasma from young mice into old mice improves stem cell function in the old animals [Villeda et al. 2014]. Rejuvenating old stem cells could therefore be an approach to enable healthy aging.
9. Altered intercellular communication
Our cells and organs do not age individually, but communicate with each other through hormones, cytokines and metabolic products. This intercellular communication plays an important role in the aging process has been shown in experiments in which the blood circulation of young and old mice was connected, an approach known as parabiosis. Old mice were partially rejuvenated by this procedure, while young mice showed signs of premature aging, suggesting that there are factors in the blood that contribute to the aging of the whole organism [Villeda et al. 2014].
When our body is aging, there are changes in signaling pathways between cells, which can potentially lead to abnormal cell function, disease development or improper response to external stimuli within the body. One of the main causes of altered cellular communication in aging is the accumulation of damage to the cells’ DNA. Altered cellular communication can lead to the accumulation of harmful proteins in the cells, which can contribute to the development of Alzheimer’s disease and other age-related conditions.
10. Inflammaging/Chronic inflammation
Inflammation is happens when our bodies try to fight off infections or heal from injuries. It’s a normal response that helps keep us healthy. But as we age, our immune system doesn’t work as well as it used to, and it can start causing problems instead of helping. This chronic low-level inflammation is called “inflammaging.” It can damage our tissues and organs, and it’s associated with age-related diseases. During aging, crosslinks form between the proteins that make up our tissues. These crosslinks glue proteins together, making our tissues stiffer. Cross-linked and glued-together collagen and elastin in the skin and blood vessels contribute to stiffer blood vessels and thus hypertension, for example.
11. Disabled macro autophagy/deteriorated autophagy
Autophagy is a kind of recycling system in human cells. It is the body’s way of breaking down unwanted and diseased cell components and recycling them elsewhere. There is strong evidence that autophagy is involved in the aging process. Studies show that the activity of autophagy-related genes decreases with age in humans [Lipinski et al. 2010]. In addition, genetic inhibition of autophagy accelerates aging in model organisms [Cassidy et al. 2020]. This could be due to an increased accumulation of proteins and cellular components, but also to a reduced ability to degrade pathogens. There is also ample evidence that stimulating autophagy increases lifespan and longevity in model organisms, highlighting the importance of autophagy in the aging process [Lu et al. 2021].
Macro autophagy is a cellular process, responsible for removing and recycling damaged cell components. When this process is disrupted, it can lead to the accumulation of waste in our cells, potentially contributing to diseases, including neurodegenerative conditions and metabolic disorders.
12. Microbiome dysbiosis/Imbalance of the intestinal flora (dysbiosis)
The human body is colonised by trillions of microorganisms, including bacteria, fungi, protists and viruses, collectively known as the microbiome. It is estimated that for every human cell there is at least one non-human cell in our bodies.
Microorganisms live on our skin and in our body fluids, but the majority are found in our digestive tract and are therefore referred to as the gut microbiome. The gut microbiome has important functions for our bodies: microorganisms help digest food, produce essential vitamins, shape our immune system and help fight off pathogens. The composition of the gut microbiome is dynamic and depends on environmental factors such as diet and stress.
While young, healthy people have a complex microbiome with many different species of bacteria, diversity decreases with age, and the microbiome of older people is less complex and characterised by the presence of more pathogenic bacteria [Ghosh et al. 2022]. Interestingly, supercentenarians, contain microbes normally found only in younger people, suggesting that they have a healthier microbiome [Wilmanski et al. 2021]. Imbalance of natural microbial community, reduction of beneficial kind and overgrowth of the harmful kind leads to brain dysfunction, poor immune health, and chronic inflammation.
All the 12 hallmarks of aging are strongly interrelated among each other. For example, genomic instability (including that caused by telomere shortening) cross talks to epigenetic alterations (e.g., through the loss-of-function mutation of epigenetic modifiers such as TET2), loss of proteostasis (e.g., due to the production of mutated, misfolded proteins), disabled macroautophagy (e.g., through the capacity of autophagy to remove supernumerary centrosomes, extra nuclear chromatin, and cytosolic DNA).
At Vitallife, you can undergo diagnostics which will reveal the impact of the aging hallmarks on your health, biological age, and longevity. You can measure your pace of aging, telomere length, cellular health, mitochondrial function, and much more. Based on your results, our experience clinicians will design a personalized healthy longevity plan for you and give you specific recommendations to improve your health, reverse the hallmarks of aging, and achieve optimal longevity.
“VitalLife Scientific Wellness Center" is a leading wellness clinic in Bangkok, Thailand that offers a wide range of services to promote longevity and anti-aging.
📍 Make an appointment & more information
Add LINE: @vitallife_wellness or Click
📞Call: 02-066-8899
Our doctors can help.
Dr. Nawin Jittat
Anti-aging and Regenerative Medicine, Preventive Medicine, Gut Health, Hormone Management, Detox
M.D., ABAARM., DTM&H, AHTC
Book an appointment